Our Focus: Immunology, Bioengineering Technologies, and Health
The worlds of immunology, pharmaceutical engineering, and advanced molecular imaging have given rise to a set of tools that allow us to probe and manipulate biological systems at the tissue, molecular, and cellular level.
In our laboratory we seek to leverage these tools for addressing challenges in human and animal health such as preventing and treating infectious diseases and respiratory illnesses.
Pulmonary Immunity
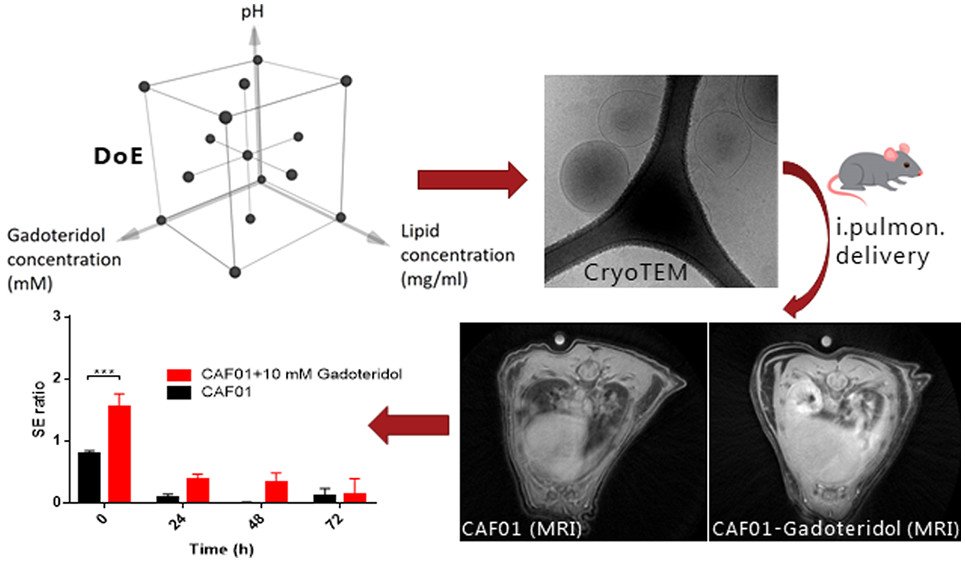
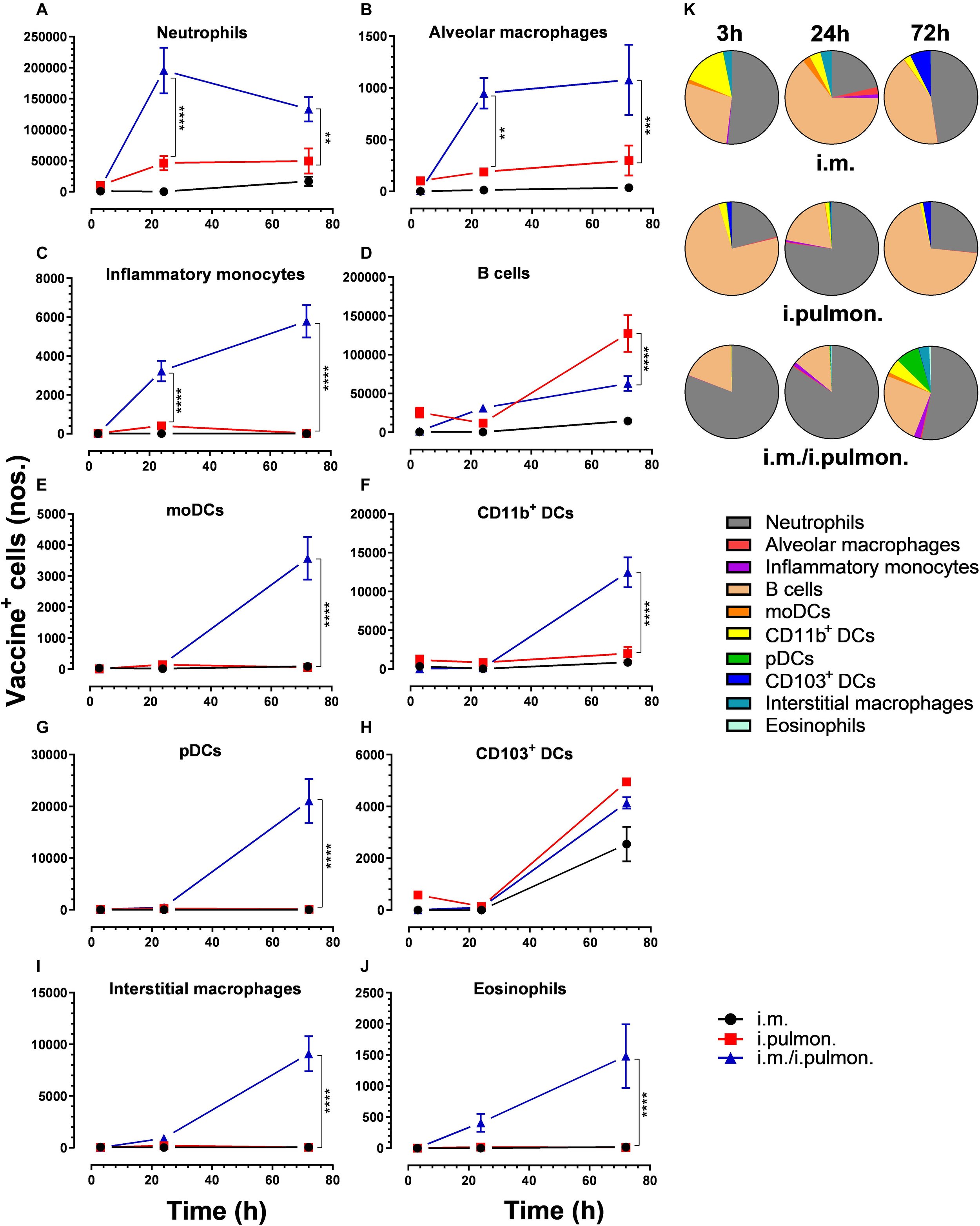
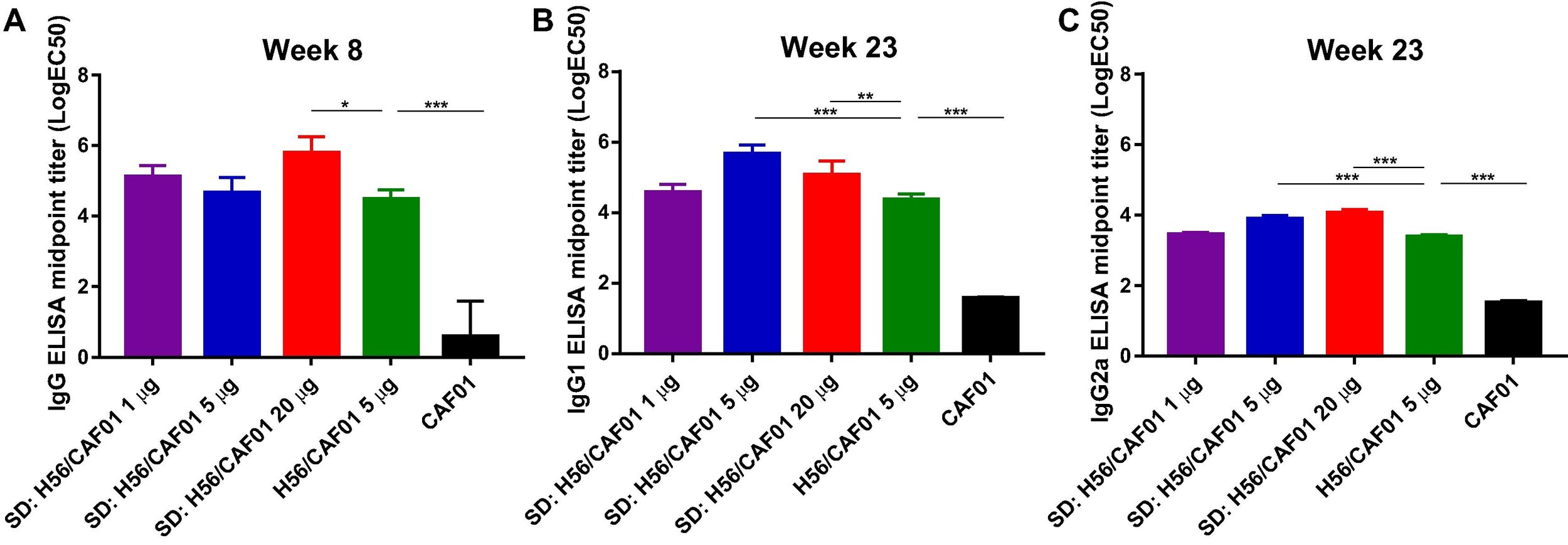
The human lung is continuously exposed to environmental and microbial antigens - both innocuous and pathogenic. Consequently, respiratory tract infections remain a leading cause of death globally. Most licensed vaccines are administered by injection and protect the host exclusively via induction of antibody responses in the blood, but they require multiple immunizations to maintain protective immunity and they do neither induce cell mediated (Th1 and CD8+ T cells) nor mucosal immunity. Thus, a major challenge of pulmonary vaccinology is to develop an approach that can provide long-lasting and durable immunity in the lungs. Mucosal vaccines have demonstrated remarkable efficacy, providing tissue-specific responses and protection across distant mucosa for improved barrier protection. However, development of effective vaccines inducing pulmonary immunity remains elusive, owing to a lack of mucosal adjuvants capable of delivering right molecular cues that yield modulated immune responses, a lack of thorough understanding of mechanisms by which lung T cells are generated and maintained in tissues during vaccination, and the optimal vaccination strategies to induce durable lung T cells are not identified. We use pharmaceutical engineering strategies and combine them with immunological principles to address these challenges.
Relevant Publications
A. Thakur, C. Rodríguez-Rodríguez, K. Saatchi, F. Rose, T. Esposito, Z. Nosrati, P. Andersen, D. Christensen, U.O. Häfeli, C. Foged
Frontiers in Immunology (2018)
A. Thakur, F. Rose, S.R. Ansari, P. Koch, V. Martini, S.L. Ovesen, B. Quistorff, S. Maritim, F. Hyder, P. Andersen, D. Christensen, Y. Mori, C. Foged
Molecular Pharmaceutics (2019)
A. Thakur, F.E. Pinto, H.S. Hansen, P. Andersen, D. Christensen, C. Janfelt, C. Foged
Frontiers in Immunology (2020)
Inhalable RNA medicines
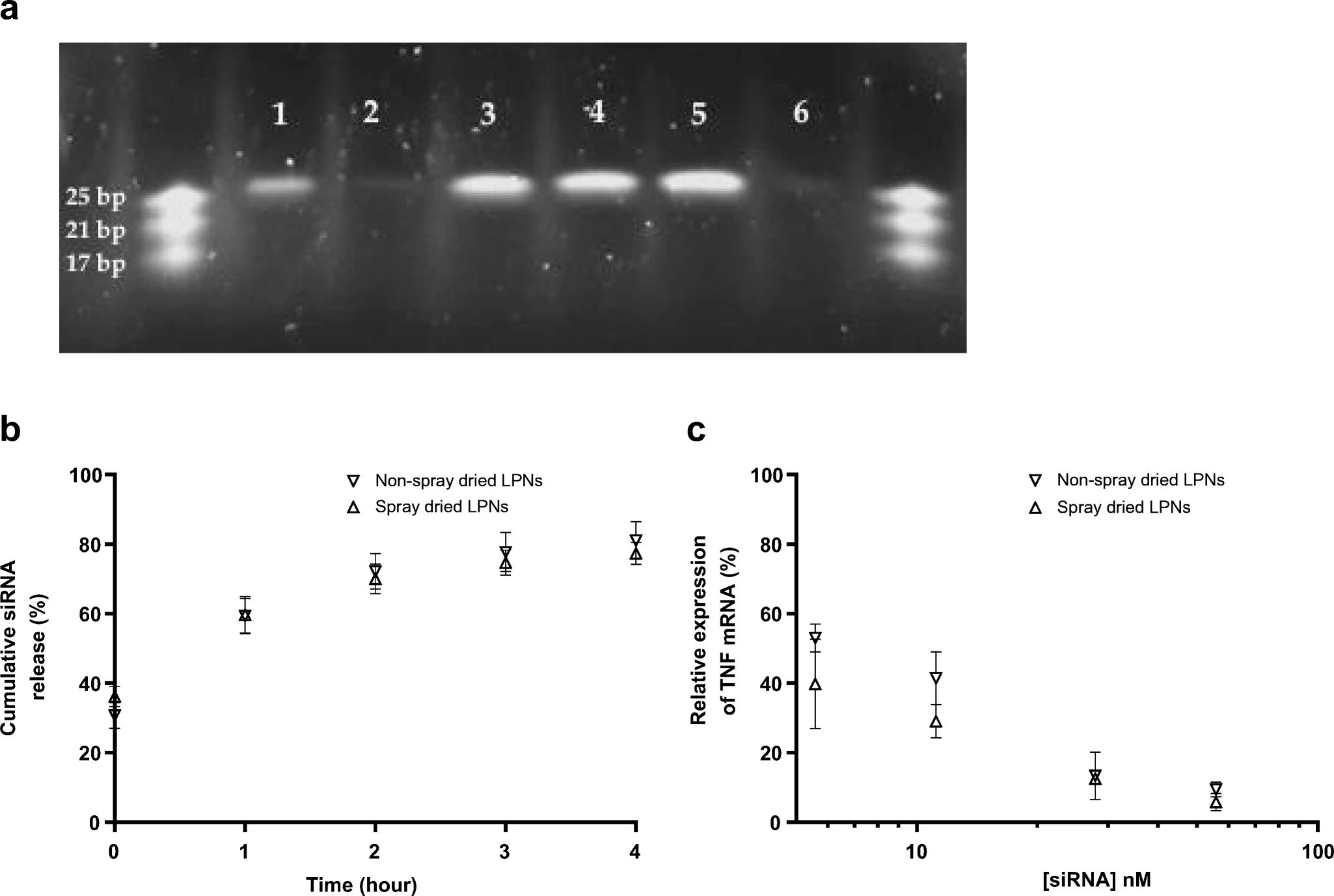
RNA molecules have great potential for treating medical conditions and preventing diseases. However, the largest impediment in the clinical translation of RNA-based therapeutics and prophylactics remains a requirement of efficient intracellular delivery of intact RNA molecules to the cells and tissue of interest without inducing adverse effects in healthy cells. Nanoparticulate drug delivery systems improve RNA stability, facilitate internalization, and increase target affinity. The administration of RNA-based drugs and vaccines by inhalation allows the direct delivery of RNA molecules to the target site of action in lungs while minimizing systemic exposure. However, the exact cellular uptake mechanisms of nanoparticulate RNA delivery systems following pulmonary administration are not completely clear. Similarly, the long-term safety of inhaled RNA therapy formulations is not well characterized. We approach these research questions by using inhalable dosage forms of therapeutic and prophylactic RNA for delineating their precise cellular uptake mechanisms.
Relevant Publications
C. Dormenval, A. Lokras, G. Cano-Garcia, A. Wadhwa, K. Thanki, F. Rose, A. Thakur, H. Franzyk, C. Foged
Pharmaceutical Research (2019)
A. Lokras, A. Thakur, A. Wadhwa, K. Thanki, H. Franzyk, C. Foged
Frontiers in Bioengineering and Biotechnology (2021)
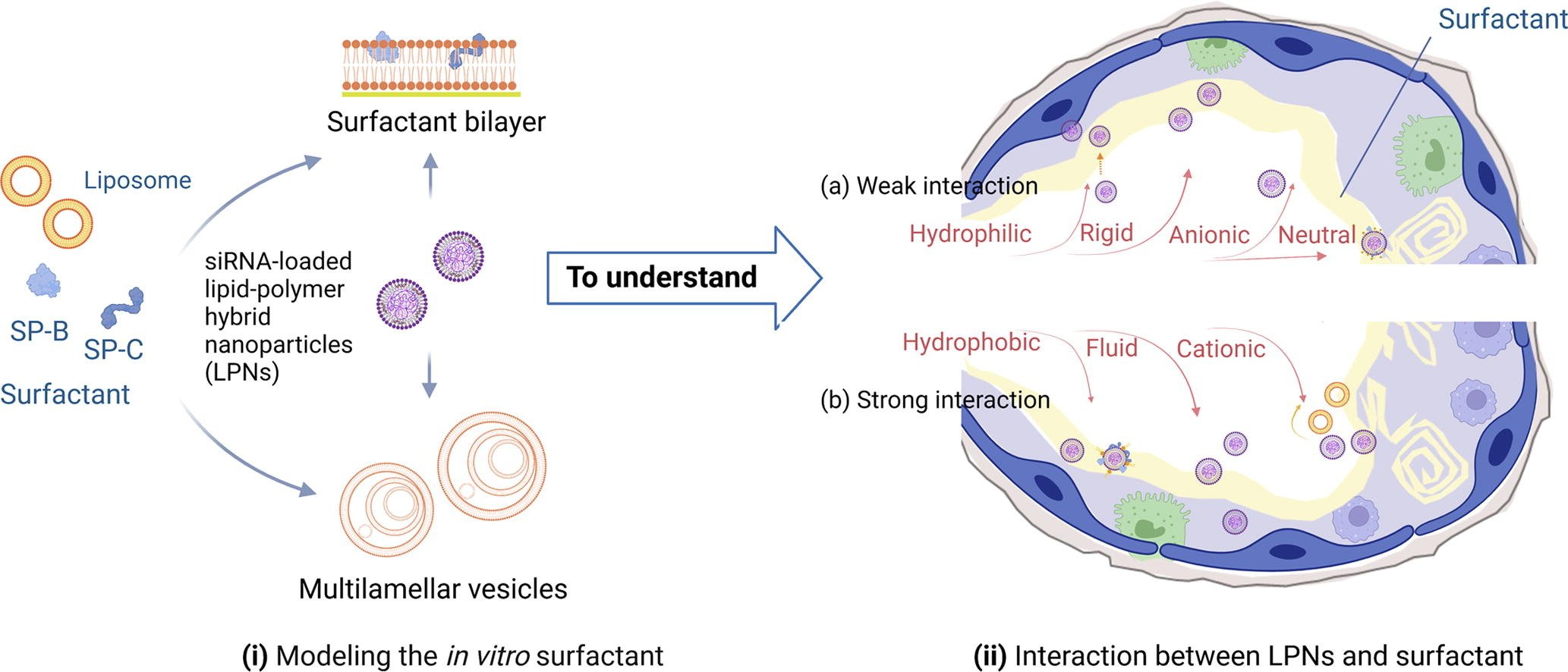
Y. Xu, E. Parra-Ortiz, F. Wan, O. Cañadas, B. Garcia-Alvarez, A. Thakur, H. Franzyk, J. Pérez-Gil, M. Malmsten, C. Foged
Journal of Colloid and Interface Science (2023)
Particle engineering
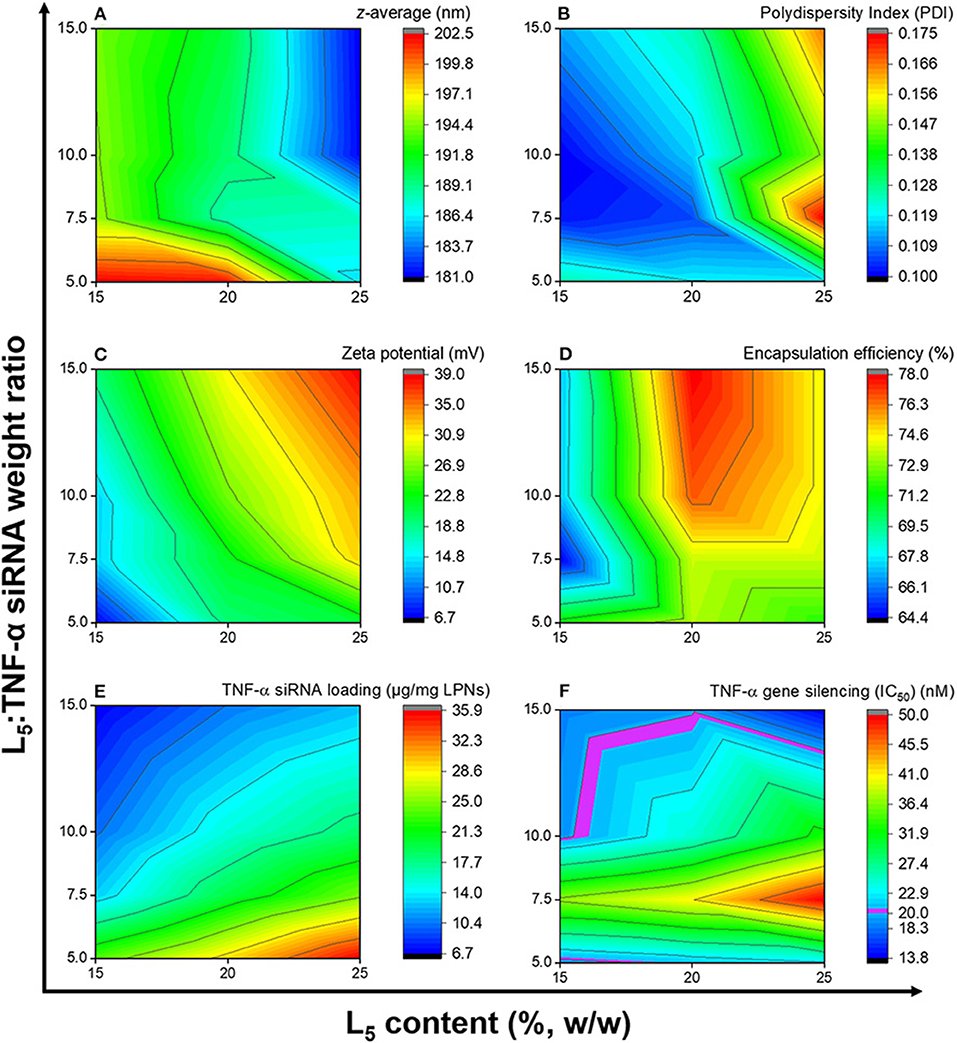
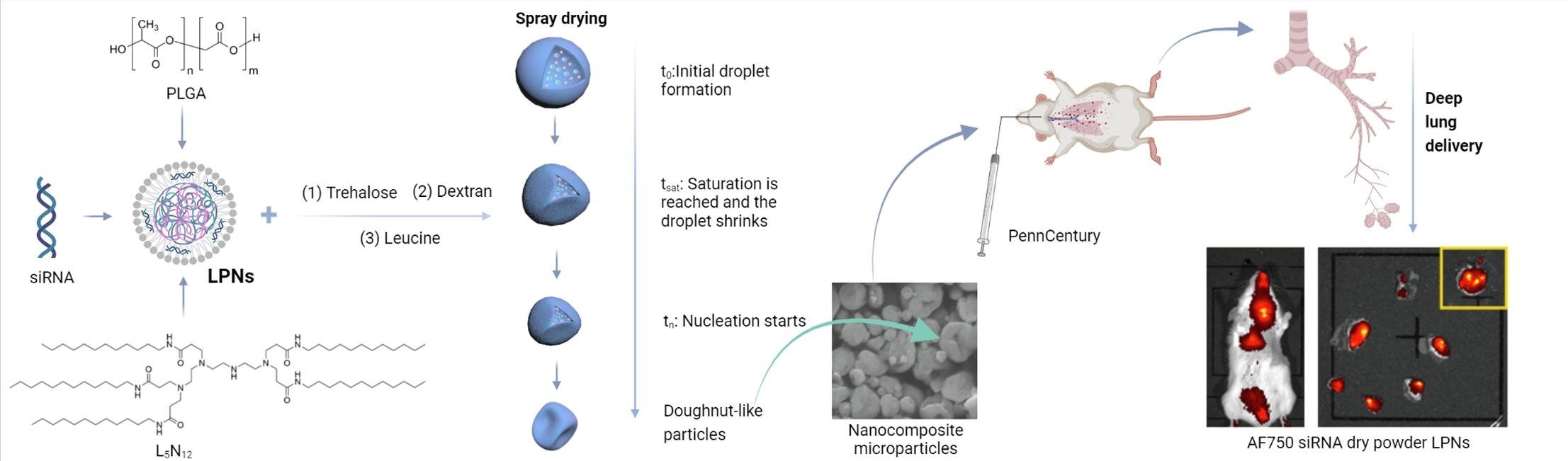
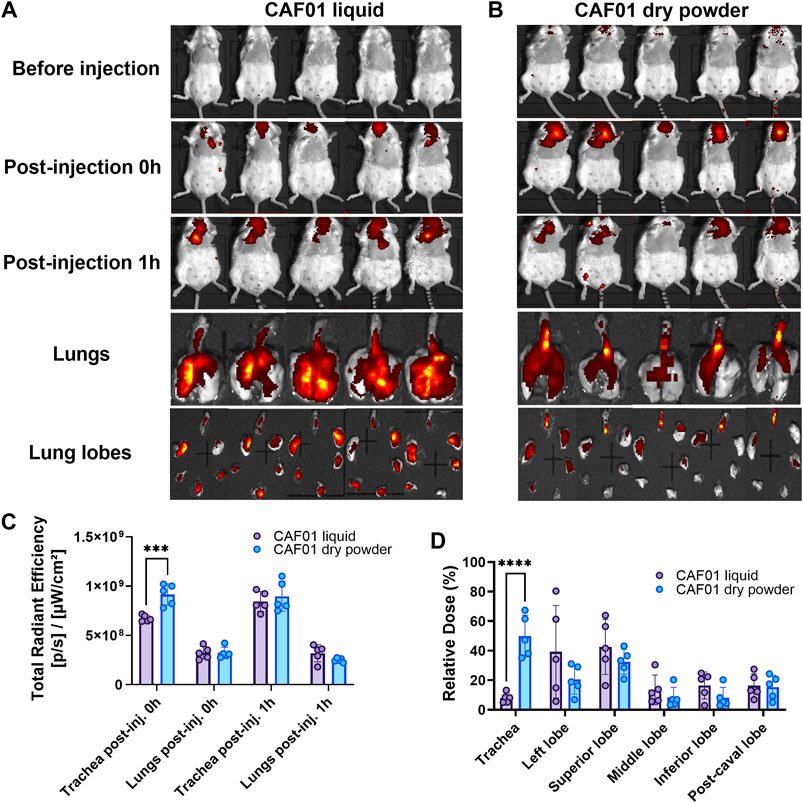
Thermostability is one of the product characteristics preferred by WHO for vaccines and biologics against respiratory infections due to ease of administration, pain minimization, and low costs. One method of improving the thermostability of biologics is through drying of a liquid formulation into a dry powder inhaler (DPI) form. Drying vaccines has the potential to extend shelf lives and increase formulation stability at high temperatures. Spray drying is one of the several drying methods that has been used to stabilize formulations into a DPI form for storage above refrigerated temperatures and it allows engineering of particles of desired configuration. To capitalize on DPI spray-dried formulations, it is necessary to resolve issues around the development process, e.g., the most optimal combination of parameters during the spray-drying process, the most suitable excipient during the drying process or the development of a dry powder carrier platform that can stabilize the biologic in a dry solid state. We work on these challenges to engineer the particles in DPI formulations for inhalation.
Relevant Publications
A. Thakur, P.T. Ingvarsson, S.T. Schmidt, F. Rose, P. Andersen, D. Christensen, C. Foged
Vaccine (2018)
Y. Xu, L. Harinck, A.G. Lokras, P. Gerde, E. Selg, C. Sjöberg, H. Franzyk, A. Thakur, C. Foged
International Journal of Pharmaceutics (2022)
A. Thakur, Y. Xu, G. Cano-Garcia, S. Feng, F. Rose, P. Gerde, P. Andersen, D. Christensen and C. Foged
Frontiers in Drug Delivery (2022)